
WHAT IS GST?
GST i.e.Goods and Service Tax is a unified tax that replaces several indirect taxesleviedby the Central Government and the State Government(s)....
Read more
19(RE-2010)/2009-14, Dated: 14/02/2011
Guidelines for availment of input combination for pharmaceutical products manufactured through Non Infringing (NI) process
Government of India All Regional Authorities (RAs); All Commissioners of Customs; All Commissioners of Excise; Trade & Industry. Subject: Guidelines for availment of input combination for pharmaceutical products manufactured through Non Infringing (NI) process. Trade & Industry belonging to Pharmaceutical sector and the Pharmaceutical Export Promotion Council have been representing before Department of Commerce that neither the Standard Input Output Norm (SION) notified for the pharmaceutical products nor the adhoc norms ratified for such products by DGFT, allow them the actual requirement of inputs for manufacture of drugs (particularly the APIs) through Non Infringing (NI) process. Hence they have been requesting for inputs combinations based on actual consumption for such products. Sd/- (Tapan Mazumder) (Issued from F. No. 01/94/180/AA/NI process/AM11/PC-4)
|

GST i.e.Goods and Service Tax is a unified tax that replaces several indirect taxesleviedby the Central Government and the State Government(s)....
Read more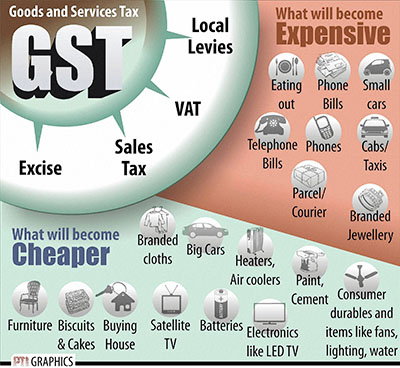
In pre-GST regime, goodswere liable to: (i) Excise Duty- on manufacture of goods; (ii) VAT/CST- on sale of goods; (iii) Entry tax- on ...
Read more
GST is levied on every taxable person. Taxable person means a person who carries on any business at any place in India. Such . ..
Read more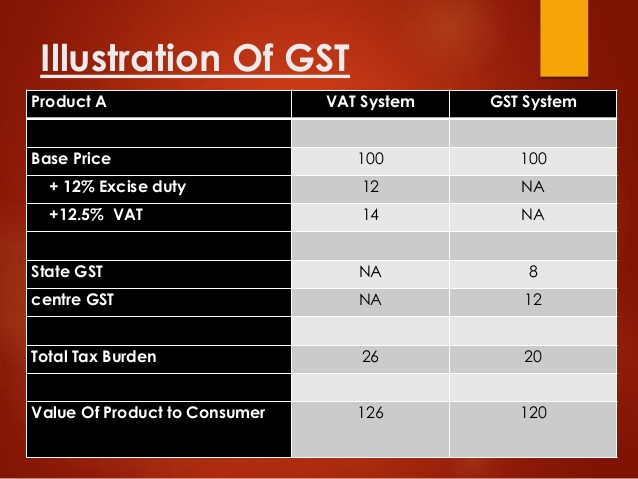
GST is a unified tax which is levied on: (i) goods; (ii) services and (iii) a mix of goods and/or services. Any supply of goods or services . .. ...
Read moreGST India Solution is an effort of firm of professionals who welcome implementation of GST. This is an interactiveplatformthat aspires to disseminate right knowledge to professionals, practitioners and public at large. This platform has beenfloatedbya firm of Chartered Accountants relentlessly working in field of direct and indirect taxes since early 1985.
READ MORE
Our core competence is statutory compliance, advisory, corporate tax planning and appellate matters of direct and indirect taxesandcorporate training sessions on GST.
The senior partner of the firm has to his credit several professional publications viz., Delhi Sales Tax Right to Use Goods Act, Delhi VAT, Maharashtra VAT, West Bengal VAT, Haryana VAT published by Taxmann. Madhya Pradesh VAT and Chhattisgarh VAT were published by Suvidha Law House, Bhopal. He has also addressed seminars on indirect taxes organized by professional bodies like ICAI, IMA, NIFM etc. and has also contributed articles on subjects of pro. . . . .